How To Make A Calibration Curve In Excel
Worksheets for Belittling Calibration Curves
Excel and OpenOffice Calc Versions
[Background] [Instructions] [Frequently Asked Questions] These are backup-the-blanks spreadsheet templates for performing the calibration bend plumbing fixtures and concentration calculations for analytical methods using the scale curve method. All you have to do is to type in (or paste in) the concentrations of the standard solutions and their musical instrument readings (e.g. absorbances, or whatever method you are using) and the instrument readings of the unknowns. The spreadsheet automatically plots and fits the data to a straight line, quadratic or cubic curve, and so uses the equation of that curve to convert the readings of the unknown samples into concentration. You can add and delete calibration points at will, to correct errors or to remove outliers; the sheet re-plots and recalculates automatically.
Screen shots and Download links:
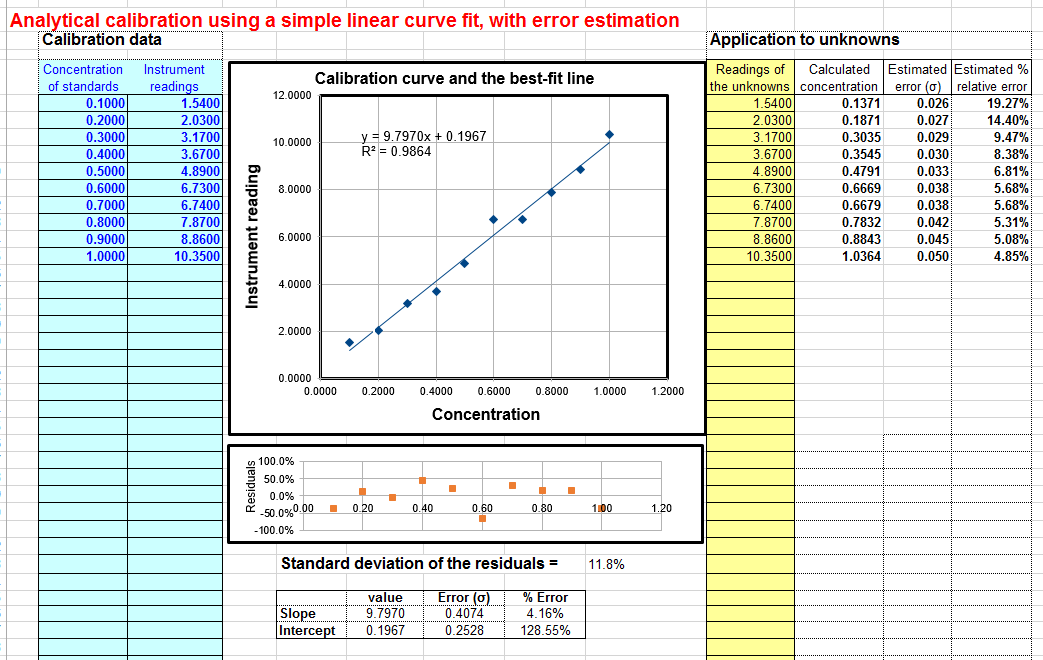 Linear calibration with error calculation. Download Excel or OpenOffice Calc format. | Linear interpolation (bracket method). Download Excel or OpenOffice Calc format | 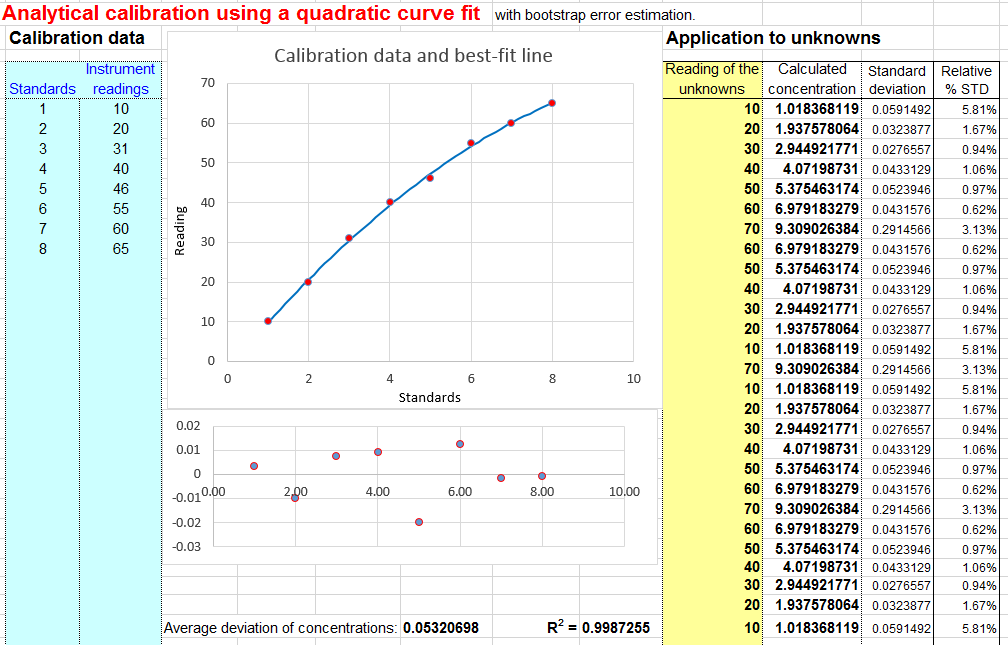 Quadratic scale with error interpretation. Download Excel or OpenOffice Calc format |
| Cubic calibration. Download Excel or OpenOffice Calc format . | 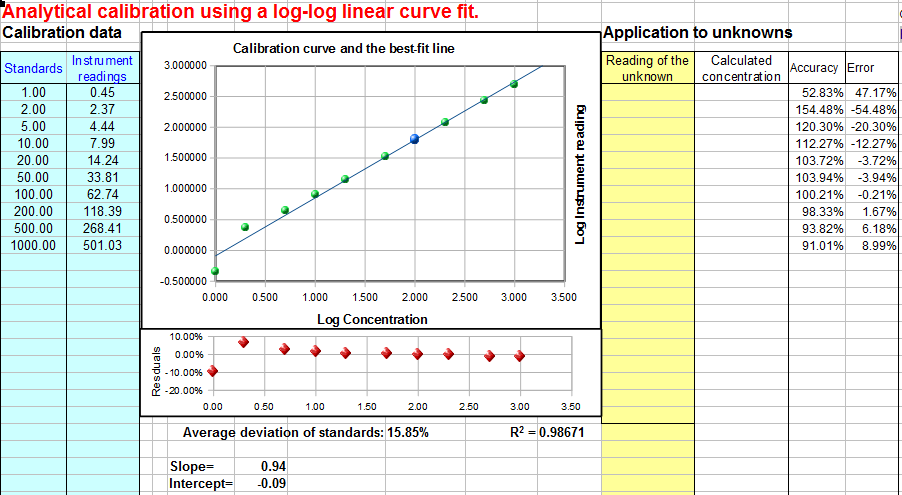 Log-log calibration. Download log-log linear (.xls or .ods) or log-log quadratic (.xls or .ods) | Drift-corrected quadratic calibration. Download Excel or OpenOffice Calc format. |
Download a ZIP file containing all the above spreadsheets (in both formats).
Note: to run these spreadsheets, y'all must take either Excel or OpenOffice Calc installed. I recommend either Excel 2013 or OpenOffice Version 4 ( download from OpenOffice).
Other related spreadsheets:
Comparison of Calibration Curve Plumbing fixtures Methods in Absorption Spectroscopy
Comparison of Analytical Calibration Methods
Instrumental Deviations from Beer's Constabulary
Multiwavelength Spectrophotometric Analysis
Transmission Fitting (TFit) Method
Curve fitting multiple overlapping peaks
Interactive Peak Fitter
Bend fitting A: Linear Least Squares
[Render to Alphabetize]
Background
In analytical chemistry, the accurate quantitative measurement of the composition of samples, for case past various types of spectroscopy, usually requires that the method exist calibrated using standard samples of known composition. This is about commonly, simply not necessarily, done with solution samples and standards dissolved in a suitable solvent, because of the ease of preparing and diluting accurate and homogeneous mixtures of samples and standards in solution form. In the calibration curve method, a series of external standard solutions is prepared and measured. A line or bend is fit to the data and the resulting equation is used to convert readings of the unknown samples into concentration. An advantage of this method is that the random errors in preparing and reading the standard solutions are averaged over several standards. Moreover, non-linearity in the calibration bend can be detected and avoided (by diluting into the linear range) or compensated (past using non-linear curve plumbing fixtures methods). In that location are worksheets here for several different calibration methods:
- A showtime-order (straight line) fit of measured betoken A (y-axis) vs concentration C (x-centrality). The model equation is A = slope * C + intercept. This is the near mutual and straightforward method, and it is the one to use if you know that your instrument response is linear. This fit is performed using the equations described and listed on http://terpconnect.umd.edu/~toh/spectrum/CurveFitting.html. You need a minimum of two points on the scale curve. The concentration of unknown samples is given by ( A - intercept) / slope where A is the measured signal and slope and intercept from the first-order fit. If you would like to use this method of calibration for your own data, download in Excel or OpenOffice Calc format. View equations for linear to the lowest degree-squares.
- Linear interpolation calibration. In the linear interpolation method (erstwhile called the subclass method), the spreadsheet performs a linear interpolation between the two standards that are just above and just below each unknown sample, rather than doing a to the lowest degree-squares fit over then entire calibration set. The concentration of the sample Cx is calculated by C1s+(C2s-C1s)*(Sx-S1s)/(S2s-S1s), where S1x and S2s are the betoken readings given by the two standards that are merely in a higher place and just below the unknown sample , C1s and C2s are the concentrations of those ii standard solutions, and Sx is the betoken given by the sample solution. This method may be useful if none of the least-squares methods are capable of plumbing equipment the entire scale range adequately (for instance, if it contains 2 linear segments with different slopes). It works well enough equally long as the standards are spaced closely enough so that the bodily signal response does not deviate significantly from linearity between the standards. However, this method does not bargain well with random besprinkle in the scale data due to random dissonance, because it does not compute a "all-time-fit" through multiple scale points as the least-squares methods do. Download a template in Excel (.xls) format.
- A quadratic fit of measured betoken A (y-axis) vs concentration C (ten-axis). The model equation is A = a C 2 + b C + c. This method can compensate for non-linearity in the instrument response to concentration. This fit is performed using the equations described and listed on http://terpconnect.umd.edu/~toh/spectrum/CurveFitting.html. You demand a minimum of iii points on the calibration bend. The concentration of unknown samples is calculated past solving this equation for C using the classical "quadratic formula",namely C = (-b+SQRT(b 2-four*a*(c-A)))/(2*a) , where A = measured signal, and a, b, and c are the three coefficients from the quadratic fit. If you would similar to use this method of calibration for your own data, download in Excel or OpenOffice Calc format. View equations for quadratic least-squares. The alternative version CalibrationQuadraticB.xlsx computes the concentration standard difference (column Fifty) and percent relative standard deviation (cavalcade M) using the bootstrap method. You need at to the lowest degree five standards for the mistake adding to work. If you go a "#NUM!" or #DIV/0" in the columns L or One thousand, just printing the F9 key to re-calculate the spreadsheet. There is also a reversed quadratic template and example, which is analogous to the reversed cubic (#five below).
- A reversed cubic fit of concentration C (y-centrality) vs measured signal A (x-axis). The model equation is C = a A 3 + b A 2 + c A + d . This method tin can compensate for more complex non-linearity that the quadratic fit. A "reversed fit" flips the usual order of axes, past plumbing fixtures concentration equally a office of measured signal. This is done in club to avoid the need to solve a cubic equation when the calibration equation is solved for C and used to convert the measured signals of the unknowns into concentration. This coordinate transformation is a short-cut, normally done in least-squares curve plumbing fixtures, at to the lowest degree by non-statisticians, to avoid mathematical messiness when the fitting equation is solved for concentration and used to convert the instrument readings into concentration values. Notwithstanding, this method is theoretically not optimum, as demonstrated for the quadratic case Monte-Carlo simulation in the spreadsheet NormalVsReversedQuadFit2.ods (Screen shot), and should be used simply if the experimental calibration curve is so non-linear that it tin not be fit at all by a quadratic - #three above.
This reversed cubic fit is performed using the LINEST role on Sheet3. You demand a minimum of four points on the calibration curve. The concentration of unknown samples is calculated directly by a A three +b A 2 +c*A +d, where A is the measured bespeak, and a, b, c, and d are the four coefficients from the cubic fit. The math is shown and explained better in the template CalibrationCubic5Points.xls (screen epitome), which is ready for a 5-point calibration, with sample data already entered. To expand this template to a greater number of calibration points, follow these steps exactly: select row nine (click on the "9" row label), right-click and select Insert, and repeat for each additional calibration point required. Then select row 8 columns D through G and drag-copy them downwardly to fill in the newly created rows. That will create all the required equations and will modify the LINEST function in O16-R20. There is also another template, CalibrationCubic.xls, that uses some spreadsheet "tricks" to automatically sense the number of scale points you enter and adjust the calculations appropriately; download in Excel or OpenOffice Calc format . - Log-log Calibration. In log-log scale, the logarithm of the measured indicate A (y-axis) is plotted against the logarithm of concentration C (x-axis) and the calibration data are fit to a linear or quadratic model, as in #i and #2 higher up. The concentration of unknown samples is obtained past taking the logarithm of the instrument readings, computing the respective logarithms of the concentrations from the scale equation, then taking the anti-log to obtain the concentration. (These additional steps practice not introduce any additional mistake, because the log and anti-log conversions can exist made apace and without significant error by the computer). Log-log calibration is well suited for information with very large range of values because it distributes the relative fitting error more than evenly amid the calibration points, preventing the larger scale points to dominate and cause excessive errors in the depression points. In some cases (e.g Power Law relationships) a nonlinear human relationship between signal and concentration can be completely linearized past a log-log transformation. However, because of the use of logarithms, the data set tin can not contain whatever zero or negative values. To employ this method of scale for your own data, download the templates for log-log linear (Excel or Calc) or log-log quadratic (Excel or Calc).
- Drift-corrected calibration. All of the in a higher place methods assume that the calibration of the instrument is stable with time and that the calibration (unremarkably performed earlier the samples are measured) remains valid while the unknown samples are measured. In some cases, however, instruments and sensors can drift, that is, the gradient and/or intercept of their calibration curves can gradually change with time after the initial calibration. Yous can examination for this drift past measuring the standards again after the samples are run, to make up one's mind how unlike the 2nd calibration curve is from the first. If the difference is not too big, it'southward reasonable to assume that the migrate is approximately linear with time, that is, that the scale curve parameters (intercept, slope, and curvature) have changed linearly as a part of fourth dimension between the two scale runs. It'south then possible to correct for the drift if you lot record the time when each calibration is run and when each unknown sample is measured. The migrate-correction spreadsheet (CalibrationDriftingQuadratic.ods) does the calculations: it computes a quadratic fit for the pre- and post-calibration curves, then uses linear interpolation to guess the calibration bend parameters for each separate sample based on the time it was measured. The method works perfectly merely if the migrate is linear with time (a reasonable assumption if the amount of migrate is not too large), but in whatever case it is better than simply bold that there is no drift at all. If you would like to use this method of scale for your own data, download in Excel or OpenOffice Calc format . (Come across Instructions: #8).
- Error calculations. In many cases it is important to summate the likely error in the computed concentration values (column K) caused by imperfect calibration. This is discussed on "Reliability of curve plumbing equipment results". The linear calibration spreadsheet (download in Excel or OpenOffice Calc format) performs a classical algebraic fault-propagation calculation on the equation that calculates the concentration from the unknown signal and the slope and intercept of the scale curve. The quadratic calibration spreadsheet ( Download in Excel or OpenOffice Calc format ) performs a bootstrap calculation . You must take a least 5 calibration points for these error calculations to be even minimally reliable; the more the ameliorate. That is because these methods need a representative sample of deviations from the ideal calibration line. If the calibration line fits the points exactly, then the computed error volition be goose egg.
Instructions:
i. Download and open the desired calibration worksheet from amongst those listed above.2. Enter the concentrations of the standards and their instrument readings (e.g. absorbance) into the blue table on the left. Get out the rest of the table blank. Yous must have at least two points on the scale curve (three points for the quadratic method or 4 points for the cubic method), including the blank (zero concentration standard). If you take multiple musical instrument readings for one standard, it's amend to enter each as a separate standard with the same concentration, rather than entering the boilerplate. The spreadsheet automatically gives more weight to standards that have more than one reading.
three. Enter the instrument readings (e.g. absorbance) of the unknowns into the yellowish table on the right. You can have any number of unknowns up to 20. (If you lot have multiple musical instrument readings for one unknown, information technology's better to enter each as a separate unknown, rather than averaging them, so you can see how much variation in calculated concentration is produced by the variation in musical instrument reading).
iv. The concentrations of the unknowns are automatically calculated and displayed column 1000. If you edit the calibration curve, by deleting, changing, or adding more calibration standards, the concentrations are automatically recalculated.
For the linear fit (CalibrationLinear.xls), if you have iii or more than calibration points, the estimated standard divergence of the gradient and intercept will be calculated and displayed in cells G36 and G37, and the resulting standard divergence (SD) of each concentration volition be displayed in rows L (absolute SD) and M (per centum relative SD). These standard deviation calculations are estimates of the variability of slopes and intercepts yous are probable to get if y'all repeated the calibration over and over multiple times under the same weather condition, assuming that the deviations from the straight line are due to r andom variability and not systematic fault acquired by not-linearity. If the deviations are random, they will exist slightly different from time to time, causing the slope and intercept to vary from measurement to measurement.. Notwithstanding, if the deviations are caused past systematic non-linearity, they will exist the same from from measurement to measurement, in which instance these predictions of standard deviation will not be relevant, and you would exist better off using a.polynomial fit such every bit a quadratic or cubic. The reliability of these standard deviation estimates as well depends on the number of data points in the curve fit; they improve with the square root of the number of points.
5. You lot tin remove any point from the bend fit past deleting the respective Ten and Y values in the table. To delete a value; right-click on the jail cell and click "Delete Contents" or "Clear Contents". The spreadsheet automatically re-calculates and the graph re-draws; if information technology does not, press F9 to recalculate. (Notation: the cubic calibration spreadsheet must have contiguous calibration points with no bare or empty cells in the calibration range).
6. The linear scale spreadsheet also calculates the coefficient of determination, R2, which is an indicator of the "goodness of fit", in cell C37. R2 is 1.0000 when the fit is perfect but less than that when the fit is imperfect. The closer to 1.0000 the ameliorate.
7. A "residuals plot" is displayed but below the calibration graph (except for the interpolation method). This shows the divergence between the best-fit calibration curve and the actual readings of the standards. The smaller these errors, the more than closely the curve fits the scale standards. (The standard deviation of those errors is also calculated and displayed below the residuals plot; the lower this standard deviation, the better).
You can tell a lot by looking at the shape of the residual plot: if the points are scattered randomly above and below zero, it means that the curve fit is equally expert every bit it can exist given the random dissonance in the information. But if the remainder plot has a smooth shape, say, a U-shaped curve, then it ways that there is a mismatch betwixt the curve fit and the bodily shape of the calibration curve; suggesting that the another bend fitting techniques might be tried (say, a quadratic or cubic fit rather than a linear one) or that the experimental weather exist modified to produce a less complex experimental calibration bend shape.
8. If yous are using the spreadsheet for drift-corrected calibration, you must mensurate two scale curves, 1 before and one after the samples are run, and record the engagement and time each calibration curve is measured. Enter the concentrations of the standards into cavalcade B. Enter the instruments readings for the starting time (pre-) calibration into cavalcade C and the date/time of that scale into cell C5; enter the instruments readings for the post-calibration into column D and the engagement/fourth dimension of that scale into cell D5. The format for the appointment/time entry is Calendar month-Day-Year Hours:Minutes:Seconds, for example 6-2-2011 13:xxx:00 for June ii, 2011, 1:xxx PM (13:30 on the 24-hr clock). Note: if both calibrations are run on the same day, you can leave off the date and just enter the time. In the graph, the pre-scale curve is shown in green and the post-calibration curve is shown in red . Then, for each unknown sample measured, enter the appointment/time (in the aforementioned format) into column G and the instrument reading for that unknown into column Fifty. The spreadsheet computes the drift-corrected sample concentrations in cavalcade Grand. Notation: Version ii.1 of this spreadsheet (July, 2011) allows different sets of concentrations for the pre- and mail-calibrations. Just list all he concentrations used in the "Concentration of standards" column (B) and put the corresponding instrument readings in columns C or D, or both. If you don't use a particular concentration for one of the calibrations, just leave that musical instrument reading blank.

Click to see larger figure
This figure shows an application of the migrate-corrected quadratic calibration spreadsheet. In this demonstration, the calibrations and measurements were made over a period of several days. The pre-scale (column C) was performed with half dozen standards (cavalcade B) on 01/25/2011 at 1:00 PM. Eight unknown samples were measured over the following five days (columns 50 and Thou), and the post-calibration (column D) was performed after then last measurement on 01/xxx/2011 at 2:45 PM. The graph in the center shows the pre-calibration curve in green and the post-calibration curve in red. Every bit you tin can see, the sensor (or the instrument) had drifted over that time period, the sensitivity (gradient of the calibration curve) becoming smaller and curve becoming noticeably more non-linear (concave downwardly). However, both the pre- and post-calibration curves fit the quadratic calibration equations very well, every bit indicated by the residuals plot and the coefficients of determination (R 2 ) listed below the graphs. The eight "unknown" samples that were measured for this examination (yellow tabular array) were really the aforementioned sample measured repeatedly - a standard of concentration 1.00 units - simply you tin run across that the sample gave lower instrument readings (cavalcade 50) each time it was measured (cavalcade K), due to the drift. Finally, the drift-corrected concentrations calculated by the spreadsheet (column M on the right) are all very close to 1.00, showing that the drift correction works well, within the limits of the random noise in the instrument readings and subject to the supposition that the drift in the scale curve parameters is linear with time between the pre- and mail service-calibrations.
Ofttimes Asked Questions (taken from actual search engine queries)
ane. Question: What is the the purpose of calibration curve? Answer: Almost analytical instruments generate an electric output signal such as a electric current or a voltage. A calibration curve establishes the relationship between the betoken generated by a measurement instrument and the concentration of the substance being measured. Dissimilar chemical compounds and elements give dissimilar signals. When an unknown sample is measured, the signal from the unknown is converted into concentration using the calibration curve.
2. Question:How do y'all make a calibration curve?
Respond: You prepare a serial of "standard solutions" of the substance that you intend to measure, measure out the point (east.g. absorbance, if you lot are doing absorption spectrophotometry), and plot the concentration on the ten-centrality and the measured signal for each standard on the y-axis. Draw a straight line as close as possible to the points on the scale curve (or a shine curve if a straight line won't fit), so that equally many points as possible are correct on or close to the curve.
3. Question: How do you lot use a scale curve to predict the concentration of an unknown sample? How exercise you lot determine concentration from a non-linear scale plot?
Answer: This can exist done in two means, graphically and mathematically. Graphically, describe a horizontal line from the bespeak of the unknown on the y axis over to the calibration curve and and then direct downward to the concentration (x) axis to the concentration of the unknown. Mathematically, fit an equation to the calibration data, and solve the equation for concentration as a role of betoken. Then, for each unknown, merely plug its signal into this equation and calculate the concentration. For example, for a linear equation, the curve fit equation is Signal = slope * Concentration + intercept, where slope and intercept are determined by a linear (outset guild) least squares curve fit to the calibration data. Solving this equation for Concentration yields Concentration = (Betoken - intercept) / slope , where Signal is the signal reading (e.g. absorbance) of the unknown solution. (Click hither for a fill-in-the-blank OpenOffice spreadsheet that does this for you . View screen shot).
4. Question: How do I know when to use a straight-line curve fit and when to use a curved line fit like a quadratic or cubic?
Answer: Fit a directly line to the scale data and look at a plot of the "residuals" (the differences between the y values in the original information and the y values computed by the fit equation). Deviations from linearity will be much more evident in the residuals plot than in the calibration curve plot. (Click hither for a fill-in-the-blank OpenOffice spreadsheet that does this for you lot . View screen shot). If the residuals are randomly scattered all along the best-fit line, so it ways that the deviations are caused by random errors such as instrument racket or by random volumetric or procedural errors; in that case you can utilise a straight line (linear) fit.
5. Question:What if my calibration curve is linear at low concentrations but curves off at the highest concentrations?
Answer: Yous can't use a linear bend fit in that case, only if the curvature is not too severe, yous might be able to go a good fit with
half-dozen. Question:What is the divergence between a scale curve and a line of best fit? What is the difference between a linear fit and a calibration curve.
Reply: The calibration curve is an experimentally measured relationship between concentration and betoken. You don't always really know the truthful calibration bend; y'all can only guess information technology at a few points by measuring a series of standard solutions. Then describe a line or a smooth curve that goes equally much every bit possible through the points, with some points being a little higher than the line and some points a little lower than the line. That's what we mean past that is a "best fit" to the information points. The actual scale bend might not be perfectly linear, then a linear fit is not e'er the all-time. A quadratic or cubic fit might be better if the calibration bend shows a gradual smooth curvature.
7. Question: Why does the slope line not go through all points on a graph?
Answer: That will only happen if you (one) are a perfect experimenter, (2) take a perfect musical instrument, and (3) choose the perfect curve-fit equation for your information. That'south not going to happen. There are always lilliputian errors. The least-squares curve-plumbing fixtures method yields a best fit, not a perfect fit, to the scale data for a given bend shape (linear. quadratic, or cubic). Points that fall off the bend are causeless to do and then because of random errors or considering the bodily scale curve shape does not match the curve-fit equation.
Actually, there is one artificial way you lot can make the curve become through all the points, and that is to employ also few scale standards: for instance, if you utilise only ii points for a direct-line fit, then the best-fit line will go right through those two points no affair what. Similarly, if you lot use only3 points for a quadratic fit, so the quadratic best-fit curve volition go right through those three points, and if you use just 4 points for a cubic fit, then the cubic best-fit curve will go right through those four points. But that's non actually recommended, because if i of your calibration points is actually off by a huge fault, the curve fit will still wait perfect, and you lot'll have no clue that something's incorrect. You actually take to use more standards that that so that yous'll know when something has gone wrong.
8. Question:What happens when the absorbance reading is higher than any of the standard solutions?
Reply: If you're using a bend-fit equation, you'll still get a value of concentration calculated for any signal reading you put in, even above the highest standard. However, information technology'due south risky to do that, because you really don't know for sure what the shape of the calibration bend is above the highest standard. It could continue direct or information technology could curve off in some unexpected way - how would yous know for sure? Information technology'due south best to add some other standard at the loftier cease of the calibration curve.
9. Question: What'southward the difference between using a single standard vs multiple standards and a graph?
Answer: The single standard method is the simplest and quickest method, but information technology is accurate just if the calibration bend is known to be linear. Using multiple standards has the advantage that any non-linearity in the calibration bend can be detected and avoided (by diluting into the linear range) or compensated (by using non-linear curve fitting methods). Also, the random errors in preparing and reading the standard solutions are averaged over several standards, which is better than "putting all your eggs in one basket" with a unmarried standard. On the other hand, an obvious disadvantage of the multiple standard method is that information technology requires much more than time and uses more standard fabric than the single standard method.
ten. Question: What's the relationship betwixt sensitivity in analysis and the gradient of standard curve?
Reply: Sensitivity is divers equally the gradient of the standard (scale) bend.
11. Question: How do you make a calibration curve in Excel or in OpenOffice?
Reply: Put the concentration of the standards in one column and their signals (e.g. absorbances) in another column. And so make an XY besprinkle graph, putting concentration on the Ten (horizontal) centrality and signal on the Y (vertical) centrality. Plot the data points with symbols only, not lines between the points. To compute a least-squares curve fit, you can either put in the least-squares equations into your spreadsheet, or y'all can use the built-in LINEST function in both Excel and OpenOffice Calc to compute polynomial and other curvilinear least-squares fits. For examples of OpenOffice spreadsheets that graphs and fits scale curves, come across
12. Question:What'southward the difference in using a calibration bend in absorption spectrometry vs other analytical methods such a fluorescence or emission spectroscopy?
Answer: The but divergence is the units of the signal. In assimilation spectroscopy y'all use absorbance (because it's the about well-nigh linear with concentration) and in fluorescence (or emission) spectroscopy you utilise the fluorescence (or emission) intensity, which is commonly linear with concentration (except sometimes at high concentrations). The methods of curve fitting and calculating the concentration are basically the aforementioned.
13. Question:If the solution obeys Beer'south Police force, is it amend to use a calibration curve rather than a single standard?
Answer: Information technology might not brand much departure either style. If the solution is known from previous measurements to obey Beer's Police exactly on the same spectrophotometer and under the atmospheric condition in use, then a single standard tin can be used (although information technology's all-time if that standard gives a signal close to the maximum expected sample indicate or to whatever signal gives the best signal-to-dissonance ratio - an absorbance near 1.0 in absorption spectroscopy). The only real reward of multiple standards in this case is that the random errors in preparing and reading the standard solutions are averaged over several standards, just the same consequence tin can exist achieved more just by making up multiple copies of the same single standard (to average out the random volumetric errors) and reading each separately (to average out the random signal reading errors). And if the signal reading errors are much smaller than the volumetric errors, then a unmarried standard solution can be measured repeatedly to average out the random measurement errors.
14. Question:What is the effect on concentration measurement if the monochromator is not perfect?
Answer: If the wavelength scale if off a little chip, it will have no significant effect every bit long as the monochromator setting is left untouched betwixt measurement of standards and unknown sample; the slope of the calibration curve will exist different, just the calculated concentrations will be OK. (But if anything changes the wavelength between the time you measure the standards and the time you lot measure the samples, an fault will result). If the wavelength has a poor stray light rating or if the resolution is poor (spectral bandpass is too big), the calibration curve may be effected adversely. In absorption spectroscopy, stray light and poor resolution may issue in non-linearity, which requires a not-linear bend plumbing fixtures method. In emission spectroscopy, stray light and poor resolution may upshot in a spectral interferences which can result in pregnant belittling errors.
xv. Question:What does it mean if the intercept of my calibration curve fit is non zero?
Respond: Ideally the y-axis intercept of the calibration curve (the signal at zero concentration) should be zero, but there are several reasons why this might not be so. (1) If there is substantial random scatter in the calibration points above and below the best-fit line, then it'south likely that the non-zero intercept is just due to random fault. If y'all prepared some other separate set of standards, that standard bend would take different intercept, either positive or negative. There is cypher that you can do well-nigh this, unless you lot can reduce the random mistake of the standards and samples. (2) If the shape of the calibration bend does not friction match the shape of the curve fit, so information technology's very likely that you'll get a non-zip intercept every time. For case, if the calibration curve bends down as concentration increases, and you use a straight-line (linear) curve fit, the intercept will be positive (that is, the curve fit line will have a positive y-axis intercept, even if the actual calibration curve goes through zero). This is an artifact of the poor curve fit selection; if yous see that happen, try a different curve shape (quadratic or cubic). (iii) If the instrument is not "zeroed" correctly, in other words, if the instrument gives a not-aught reading when the blank solution is measured. In that case you have three choices: you lot tin cypher the instrument (if that's possible); you tin can subtract the blank signal from all the standard and sample readings; or you can just let the curve fit decrease the intercept for you (if your curve fit procedure calculates the intercept and you keep it in the solution to that equation, e.g. Concentration = (Bespeak - intercept) / slope).
16. Question:How can I reduce the random scatter of scale points above and beneath the all-time-fit line?
Respond: Random errors similar this could be due either to random volumetric errors (small errors in volumes used to prepare the standard solution by diluting from the stack solution or in adding reagents) or they may be due to random signal reading errors of the instrument, or to both. To reduce the volumetric mistake, use more precise volumetric equipment and practice your technique to perfect information technology (for example, use your technique to evangelize pure water and weigh information technology on a precise analytical rest). To reduce the betoken reading mistake, adjust the instrument conditions (e.g. wavelength, path length, slit width, etc) for best signal-to-noise ratio and average several readings of each sample or standard.
17. Question:What are interferences? What effect do interferences have on the calibration bend and on the accurateness of concentration measurement?
Answer: When an analytical method is applied to complex real-world samples, for example the determination of drugs in blood serum, measurement error tin occur due to interferences. Interferences are measurement errors caused past chemical components in the samples that influence the measured betoken, for case by contributing their own signals or past reducing or increasing the indicate from the analyte. Even if the method is well calibrated and is capable of measuring solutions of pure analyte accurately , interference errors may occur when the method is applied to complex real-world samples. Ane mode to correct for interferences is to apply "matched-matrix standards", standard solution that are prepared to contain everything that the real samples contain, except that they have known concentrations of analyte. But this is very difficult and expensive to do exactly, so every attempt is made to reduce or compensate for interferences in other ways. For more than information on types of interferences and methods to recoup for them, run across Comparison of Analytical Calibration Methods. eighteen. Question:What are the sources of mistake in preparing a scale bend?
Reply: A calibration curve is a plot of belittling signal (east.thou. absorbance, in absorption spectrophotometry) vs concentration of the standard solutions. Therefore, the main sources of error are the errors in the standard concentrations and the errors in their measured signals. Concentration errors depend mainly of the accuracy of the volumetric glassware (volumetric flasks, pipettes, solution delivery devices) and on the precision of their employ by the persons preparing the solutions. In general, the accurateness and precision of handling large volumes higher up 10 mL is greater than that at lower volumes below 1 mL. Volumetric glassware can be calibrated past weighing water on a precise analytical balance (you lot tin look up the density of water at various temperatures and thus calculate the exact volume of water from its measured weight); this would allow yous to label each of the flasks, etc, with their actual volume. Only precision may still be a problem, especially a lower volumes, and information technology's very much operator-dependent. It takes do to get good at handling small volumes. Betoken measurement fault depends hugely on the instrumental method used and on the concentration of the analyte; it can vary from about 0.1% nether ideal conditions to xxx% near the detection limit of the method. Averaging echo measurements tin can improve the precision with respect to random noise. To improve the signal-to-noise ratio at low concentrations, you may consider modifying the conditions, such as changing the slit width or the path length, or using some other instrumental method (such equally a graphite furnace atomizer rather than flame atomic absorption).
19. How can I find the error in a specific quantity using to the lowest degree foursquare fitting method? How can I judge the error in the calculated slope and intercept?
When using a simple straight-line (first gild) least-squares fit, the best fit line is specified past merely ii quantities: the slope and the intercept. The random mistake in the gradient and intercept (specifically, their standard deviation) can exist estimated mathematically from the extent to which the calibration points deviate from the all-time-fit line. The equations for doing this are given here and are implemented in the "spreadsheet for linear calibration with error calculation ".It'south important to realize that these error computations are but estimates, because they are based on the assumption that the scale data set is representative of all the scale sets that would be obtained if you repeated the calibration a large number of times - in other words, the assumption is that the random errors (volumetric and signal measurement errors) in your particular data set are typical. If your random errors happen to be small when yous run your scale curve, you'll get a deceptively good-looking calibration curve, but your estimates of the random error in the gradient and intercept volition be also low. If your random errors happen to be large, you'll get a deceptively bad-looking calibration bend, and your estimates of the random error in the gradient and intercept will be besides high. These fault estimates tin be especially poor when the number of points in a scale curve is modest; the accuracy of the estimates increases if the number of data points increases, simply of class preparing a large number of standard solutions is time consuming and expensive. The lesser line is that yous can only expect these mistake predictions from a single calibration curve to be very rough; they could hands be off by a factor of ii or more, as demonstrated by the simulation "Fault propagation in the Linear Calibration Curve Method" (download OpenOffice version). 20. How can I judge the fault in the calculated concentrations of the unknowns?
You tin can employ the slope and intercept from the least-squares fit to calculate the concentration of an unknown solution past measuring its signal and computing(Signal - intercept) / slope , where Signal is the bespeak reading (e.g. absorbance) of the unknown solution. The errors in this calculated concentration tin can then be estimated by the usual rules for the propagation of error: start, the error in (Signal - intercept) is computed by the rule for addition and subtraction; 2nd, the error in (Signal - intercept) / gradient is computed by the rule for multiplication and division. The equations for doing this are given hither and are implemented in the " spreadsheet for linear scale with mistake adding" .It's important to realize that these mistake computations are simply estimates, for the reason given in #19 above, peculiarly if the number of points in a calibration curve is pocket-size, as demonstrated by the simulation "Error propagation in the Linear Scale Curve Method" (download OpenOffice version).
21. What is the minimum adequate value of the coefficient of decision (R 2 ) ?
It depends on the accurateness required. As a rough rule of pollex, if you need an accurateness of about 0.v%, you need an R 2 of 0.9998; if a 1% mistake is skillful enough, an R 2 of 0.997 will practise; and if a 5% error is acceptable, an R ii of 0.97 will do. The bottom line is that the R ii must be pretty darned close to i.0 for quantitative results in analytical chemistry.
(c) 2008, 2021 Prof. Tom O'Haver , Professor Emeritus, The University of Maryland at College Park. Comments, suggestions and questions should exist directed to Prof. O'Haver at toh@umd.edu.
Number of unique visits since May 17, 2008. Final updated October, 2021
Source: https://terpconnect.umd.edu/~toh/models/CalibrationCurve.html

0 Response to "How To Make A Calibration Curve In Excel"
Post a Comment